Type
Sandwich ELISA, HRP-labelled antibody
Applications
Plasma-EDTA, Amniotic fluid, Cell culture supernatant
Sample Requirements
25 µl/well
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
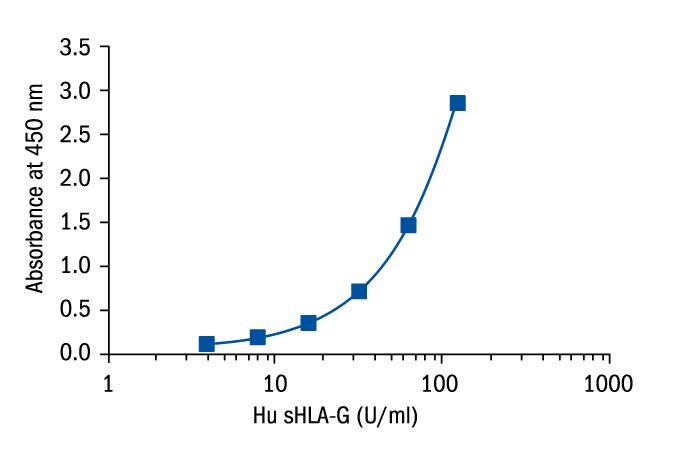
Calibration Curve
Calibration Range
3.91–125 Units/ml
Limit of Detection
0.6 Units/ml
Intra-assay (Within-Run)
n = 8; CV = 4.2%
Inter-assay (Run-to-Run)
n = 11; CV = 7.0%
Spiking Recovery
91,20%
Dilution Linearity
99,40%
Features
- It is intended for research use only
- The total assay time is about 20 hours
- The kit measures shedded HLA-G1 and HLA-G5 in EDTA plasma, amniotic fluid, or cell culture supernatant
- Calibrator is human native protein
- Assay format is 96 wells
- Components of the kit are provided ready to use, concentrated or lyophilized
Research topic
Immune Response, Infection and Inflammation, Reproduction, Transplantation
Summary
HLA-G differs from the other MHC class I genes by its low polymorphism and alternative splicing that generates seven HLA-G proteins, whose tissue-distribution is restricted to normal fetal and adult tissues that display a tolerogeneic function toward both innate and acquired immune cells. Soluble HLA-G is an immunosuppressive molecule inducing apoptosis of activated CD8(+) T cells and down-modulating CD4(+) T cell proliferation. Recently, using specific ELISA to analyse the presence of sHLA-G molecules in culture supernatants of early embryos obtained by in vitro fertilization (IVF) before transfer, several reports demonstrated that positive embryo implantations occurred with embryo secreting sHLA-G molecules. These breakthrough results indicate that sHLA-G ELISA can be a useful biochemical assay in addition to embryo morphology in embryo selection for transfer in IVF treatment if there are other embryos with the same morphology. Furthermore, monitoring of sHLA-G in amniotic fluid and plasma of pregnant women may have an important prognostic value to recognize pathological situations. Other interesting observations suggest that HLA-G molecules seem to be directly involved in transplant acceptation, and their analysis should be taken into consideration when monitoring transplant-patients status. In addition, soluble HLA-G plasma levels are increased in lyphoproliferative disorders or in patients suffering from malignant melanoma, glioma, breast and ovarian cancer.
Instructions for Use (RUO)
Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)
MSDS (RUO)
Find documents for the lot