Type
Sandwich ELISA, HRP-labelled antibody
Description
The human anti-IFN-alpha ELISA is an enzyme-linked immunosorbent assay for the quantitative
detection of human anti-IFN-alpha. The human anti-IFN-alpha ELISA is for research use only.
Applications
Serum, Urine, Plasma, Cell culture supernatant
Sample Requirements
20 µl/well
Shipping
On blue ice packs. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
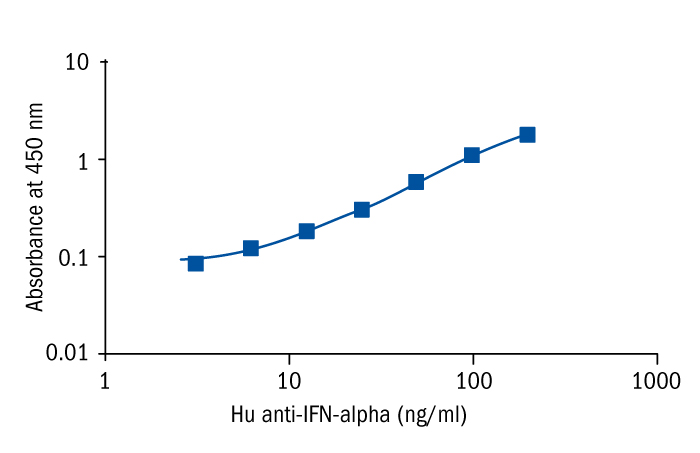
Calibration Curve
Calibration Range
3.1 –200 ng/ml
Limit of Detection
1.4 ng/ml
Intra-assay (Within-Run)
CV = 3.3%
Inter-assay (Run-to-Run)
CV = 4.1%
Spiking Recovery
92,00%
Dilution Linearity
104,00%
Features
- RUO
- sensitivity 1.4 ng/ml
- intraassay CV = 3.3%
- interassay CV = 4.1%
- standard curve: 3.1 - 200 ng/ml
Research topic
Autoimmunity, Cytokines and chemokines and related molecules, Immune Response, Infection and Inflammation
Summary
Studies on antigenicity led to the concept that molecules like the interferons were not
immunogenic in homologous systems because antibodies are not normally produced against
"self" antigens. However, naturally occuring or therapeutically induced antibodies to cytokines
such as interferons, tumor necrosis factors (TNF), interleukins (IL) and various growth factors
were found, which are generally thought to inhibit cytokine functions, and the appearance of such
antibodies should therefore result in various degrees of cytokine deficiency. It is a common
concept that the development of antibodies against any auto-antigen or drug is always
undesirable. Such antibodies are crucial for the pathology of autoimmune diseases and inhibit the
pharmacological effects of drugs including exogenously administered cytokines.
Instructions for Use (RUO)
Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)
Find documents for the lot