Research topic
Diabetology - Insulin, C-Peptide, Proinsulin, Energy metabolism and body weight regulation, Animal studies
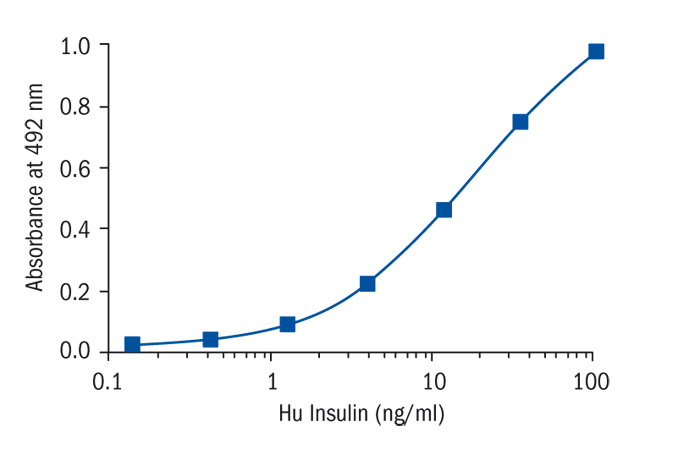
Summary
Insulin is a peptide hormone secreted from B cells of islet of Langerhans in the pancreas with a molecular weight of about 5,800 and pI 5.4. It is consisted of 2 chains, A and B. It has 3 disulfide bonds formed between A6 and A11, A7 and B7, and A20 and B19. Insulin exists as a dimer molecule in acidic to neutral solution without Zn ion, and as a hexamer including two Zn ions in neutral solution if Zn ions are present. Main targets of insulin are liver, muscle, and adipose tissue. Insulin actions in these targets are as follows. In the liver, it promotes glycogenesis, protein synthesis, fatty acid synthesis, carbohydrate utilization, and inhibition of gluconeogenesis. In the muscle, it promotes membrane permeability for carbohydrates, amino acids and K ion, glycogenesis, protein synthesis, while inhibits protein degradation. In the adipose tissue, it promotes membrane permeability for glucose and fatty acid synthesis. A precursor of insulin, called proinsulin with a single polypeptide chain, is first synthesized in the cell, then sulfide bonds are formed, and finally by enzymatic cutting at two sites, active insulin and c-peptide (connecting peptide) are formed. Potency of an insulin preparation was originally determined by bioassay. However, whole body bioassay inevitably shows poor precision owing to individual variation. WHO issued 1st International Standard for human insulin in 1986 which has the potency of 26 IU/mg (0.038 mg/IU). In the same year, 1st International Standard of bovine insulin, the potency of which is 25.7 IU/mg, and Porcine insulin 1st International Standard, 26 IU/mg, were provided. Before these standards, in 1974, 1st International Reference Preparation of human insulin for immunoassay was provided as 3 IU/ampoule. Based on the above data, if the biological activity of insulin per molecule is the same among various animal species, potencies of animal insulin might be calculated from their molecular weights. But, so far, we do not have experimental proof about this. As the molecular weights of insulin of various animals are nearly the same, and the differences are within 1%, there may be no critical fault if we think that the general potency of insulin is 26 IU/mg. Rat and mouse have two molecular species of insulin, type 1 and type 2. Amino acid sequences of these molecular species are same between rat and mouse. But as their ratios are different between these two animal species, it is recommended to use standard preparation derived from each animals.