Cat # changed from RBCS5000 to S5000
Type
Colorimetric assay
Applications
Cell culture and/or animal studies
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
All components are stable for one year, (from Invoice Date), when stored at 15–25°C. The glass vial containing collagen standard should be stored at +4°C once opened.
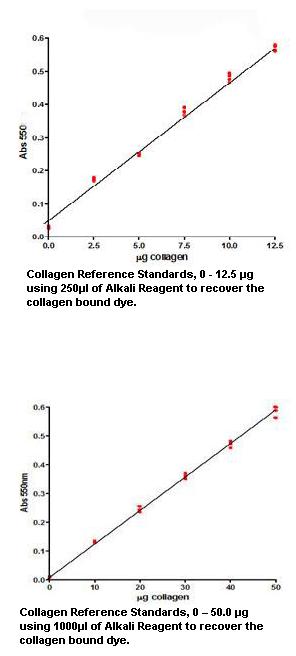
Calibration Curve
Calibration Range
2.5 – 12.5 μg using 250μl of Alkali Reagent to recover the collagen bound dye
10 – 50 μg using 1000μl of Alkali Reagent to recover the collagen bound dye
Limit of Detection
1.0 μg
Features
- The Sircol Assay is a dye-binding method designed for the analysis of acid and pepsin-soluble collagens. The assay can assess the rate of newly synthesised collagen produced during periods of rapid growth and development. New collagen is also generated during inflammation, wound healing and tumour development.
- The Sircol Assay is suitable for monitoring collagen produced in situ or during in-vitro cell culture and in-vitro extracellular matrix, (ECM), formation.
- Mammalian collagens; Types I to V can be measured. Sircol Dye reagent does not discriminate between collagen types, binding to the [Gly-X-Y]n helical structure as found in collagen.
- Not suitable for covalent cross-linked collagen. This insoluble collagen can be estimated, following acid hydrolysis, (HCl (6M), 110 °C, overnight), with an amino acid analyser to measure the collagen marker - hydroxyproline.
- Manufactured by Biocolor.
Research topic
Extracellular matrix, Animal studies
Summary
Soluble and Insoluble Collagen measurement Collagen is the most abundant protein found in animals. During a healthy life span the insoluble, covalent, cross-linked collagen fibers retain their biophysical functions and shapes. The collagen fibers remain isolated from most of the biochemical activities of the cells.
Trauma caused by metabolic internal events, external chemical agents or physical injuries however, quickly lead to dramatic activity, resulting in rapid collagen removal followed by a wound healing response (collagen regeneration and associated remodeling). Newly formed soluble collagen production and the residual insoluble collagen fibers can be monitored using the Sircol Soluble Collagen and Sircol Insoluble Collagen Assays.
Collagen proteins contain one or more domains with a triple helical structure. The three chains are described as alpha chains and should not be confused with the alpha helixes found in other proteins. The fibrillar collagens (Types I, II, III, V & XI) have most of their alpha chain structure composed of a continuous repeating tri-peptide sequence made up of glycine in every third amino acid residue [(gly-X-Y)n ]. It is to this sequence that the Sircol Dye binds.
Proline is frequently an occupant in the ‘Y’ location of the tri-peptide [(gly-X-Y)n]. Many of these residues are converted, post translation, into hydroxyproline residues prior to triple helix formation and the release of the tropocollagen into the ECM.
Until now, an investigator seeking to measure covalent cross-linked insoluble collagen required measurements based on free hydroxyproline content - a procedure that requires strong acid (6.0 M HCl), high temperatures (+ 95 °C) and overnight cooking (18 to 24 hrs).