Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum, Cell culture supernatant
Sample Requirements
100 µl (1:20 prediluted)
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
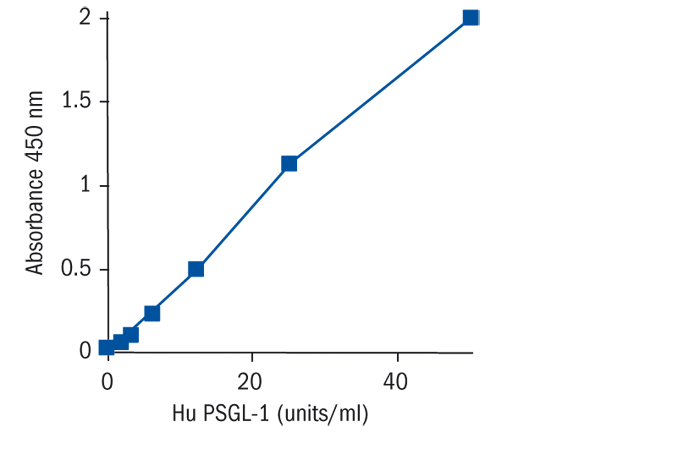
Calibration Curve
Calibration Range
1.6–50 U/ml
Limit of Detection
0.99 U/ml
Intra-assay (Within-Run)
CV = 3.2%
Inter-assay (Run-to-Run)
CV = 6.6%
Spiking Recovery
94,00%
Dilution Linearity
95,30%
Research topic
Cell adhesion proteins
Summary
The family of selectins consists of three structurally and functionally related molecules. Lselectin
is constitutively expressed on neutrophils, P-selectin is found on platelets and is stored in Weibel-Palade bodies from where it is transported to the cell surface upon endothelial activation. E-selectin is expressed on endothelial cells. Due to a common structural element, the amino-terminal lectin-like domain, the selectins are able to bind to carbohydrate ligands.
Different putative ligand structures have been identified for which the selectins show high affinity. These structures include oligosaccharides, phophorylated saccharides, sulfopolysaccharides and lipids.
It was shown that glycoproteins represent the biological relevant ligands for selectins. While the ligands for E- and L-selectins with primary binding activity have not been identified so far, the functionally most important ligand for P-selectin has been identified. The mucin-like glycoprotein PSGL-1 (P-selectin Glycoprotein Ligand-1) has been cloned and sequenced. PSGL-1 has been shown to be a transmembrane protein which forms homodimers via disulfide bridges of two 120 kDa chains.
PSGL-1 is expressed on cells of myeloid, lymphoid and dendritic lineage. The binding of Pselectin is regulated by different degrees and forms of glycosylation. An interaction of Lselectin with PSGL-1 in the process of neutrophil aggregation has been shown. However, PSGL-1 does not seem to be the primary ligand for L-selectin. Presently it is not known which cells, apart from leukocytes, express PSGL-1 and what role
PSGL-1 plays on these cells. The metastic potential of the majority of cells which bind to Pselectin is however in close correlation with the functional expression of PSGL-1 on these cells. The regulation of PSGL-1 is not yet well described. Glycosyltransferases sure play an important role in activation. The deactivation of PSGL-1 is so far unclear. The cleavage of the protein from the cell surface is one mechanism involved in the deactivation process. Following this shedding, a soluble form of PSGL-1 is detectable in the circulation. This soluble
isoform of PSGL-1 is still capable of binding to P-selectin, thus representing a competitor for cellular PSGL-1 through which regulation in many physiological and pathological processes can take place.
Instructions for Use (RUO)
Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)
Find documents for the lot