Type
Recombinant protein
Description
Total 404 AA. MW: 45.9 kDa (calculated). UniProtKB acc.no. Q99574 (Thr17-Leu410). N-Terminal His-tag (10 extra AA). Protein identity confirmed by MS.
Amino Acid Sequence
MKHHHHHHASTGATFPEEAIADLSVNMYNRLRATGEDENILFSPLSIALAMGMMELGAQGSTQKEIRHSMGYDSLKNGEEFSFLKEFSNMVTAKESQYVMKIANSLFVQNGFHVNEEFLQMMKKYFNAAVNHVDFSQNVAVANYINKWVENNTNNLVKDLVSPRDFDAATYLALINAVYFKGNWKSQFRPENTRTFSFTKDDESEVQIPMMYQQGEFYYGEFSDGSNEAGGIYQVLEIPYEGDEISMMLVLSRQEVPLATLEPLVKAQLVEEWANSVKKQKVEVYLPRFTVEQEIDLKDVLKALGITEIFIKDANLTGLSDNKEIFLSKAIHKSFLEVNEEGSEAAAVSGMIAISRMAVLYPQVIVDHPFFFLIRNRRTGTILFMGRVMHPETMNTSGHDFEEL
Source
E. coli
Purity
˃ 90 % by SDS-PAGE
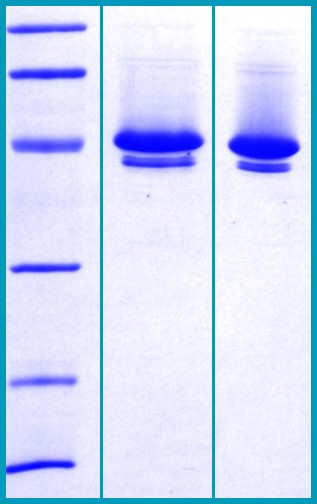
SDS-PAGE Gel
12% SDS-PAGE separation of Human Neuroserpin:
1. MW marker – 97, 66, 45, 31, 21, 14 kDa
2. non reduced and non boiled sample, 2.5 μg / lane
3. reduced and boiled sample, 2.5 μg / lane
Endotoxin
< 1.0 EU/µg
Formulation
Filtered (0.4 μm) and lyophilized in 0.5 mg/mL in 0.025M phosphate buffer, 0.035M NaCl, pH 7.4
Reconstitution
Add 200ul of deionized water to prepare a working stock solution of 0.5 mg/mL and let the lyophilized pellet dissolve completely.
Applications
Western blotting, ELISA
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store protein at -80°C. Protein remains stable until the expiry date when stored at -80°C. Avoid repeated freezing/thawing cycles. Defrosted protein can be stored at 4°C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
Endotoxin level determination.
Note
This product is intended for research use only.
Research topic
Neural tissue markers, Oncology
Summary
Neuroserpin (NSP; alternative names: serpin I1, peptidase inhibitor 12, PI-12) is a secreted glycoprotein which consists of 410 amino acid residues (46.4 kDa – if taken without glycosylation) including 16 amino acids of potential signal peptide. Neuroserpin-coding gene (PI12) consists of 9 exons and 8 introns and has a strongly conserved gene organization. Neuroserpin is a serine protease inhibitor of the serpin family that has been identified as an axonally secreted glycoprotein in neuronal cultures of chicken dorsal root ganglia. Neuroserpin-coding gene was later identified also in human; it was cloned, expressed and characterised. Human neuroserpin exhibits high amino acid identity with some other neuroserpins from mammals, e.g. 88%, 86% and 80% identity with neuroserpin of rat, mouse and chicken, respectively. Two secreted forms were observed with molecular weights of 49 and 50 kDa which may represent alternative glycosylation at three putative N-linked glycosylation sites. PI12 transcript (neuroserpin mRNA) was observed mainly in the brain, where the gene is ubiquitously expressed. Expression of neuroserpin was also detected in the spinal cord and, with much weaker signals, in some other tissues such as pancreas, heart, kidney, testis and placenta. NSP mRNA was detected extensively in normal brain tissue, but not in brain tumors. Neuroserpin has been suggested to be a critical regulator of tissue-type plasminogen activator (tPA) activity in the central nervous system, and as such may play an important role in neuronal plasticity and/or maintenance. Expression of tPA in some brain regions (choroid plexus, Purkyně cells of the cerebellum, discrete neuron areas of the hypothalamus and hippocampus, and in the myelinated axons of the commissura) is reported to be high and has previously been correlated with both motor learning and neuronal survival. Tissue plasminogen activator (tPA) is commonly used as a thrombolytic agent to remove fibrin blood clots but it has also been directly implicated in neurovascular breakdown in ischemic stroke. It was suggested that neuroprotective properties of neuroserpin, the inhibitor of tPA, may be related to the inhibition of excitotoxicity, inflammation, as well as blood brain barrier disruption that occur after acute ischemic stroke. Some point mutations in the neuroserpin gene cause familial encephalopathy with neuroserpin inclusion bodies (FENIB). FENIB is characterized by intraneuronal accumulation of neuroserpin polymers and consequent dementia with different age of onset, ranging from before age of 20 years to old age. To date, six autosomal dominantly inherited mutations have been described. It has been shown that neuroserpin is a plaque-associated protein in brains of patients with Alzheimer disease and that it forms a specific binary complex with Aß1–42 peptide which is also characteristic for Alzheimer disease (AD). The neuroprotective nature of this interaction has been indicated in cell culture and on Drosophila models of disease. Higher CSF levels of neuroserpin together with α1-antichymotrypsin have been shown to be associated with the clinical diagnosis of Alzheimer disease vs healthy controls. The neuroserpin gene was reported among genes which are overexpressed at some types of cancer – e.g. hepatocellular cancer and prostatic cancer.
Product Datasheet (RUO)
Datasheet PDF (RUO)
Datasheet PDF (RUO)
Safety Information (RUO)
MSDS (RUO)
Find documents for the lot