Type
Sandwich ELISA, Biotin-labelled antibody
Applications
Serum
Sample Requirements
10 µl/well
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
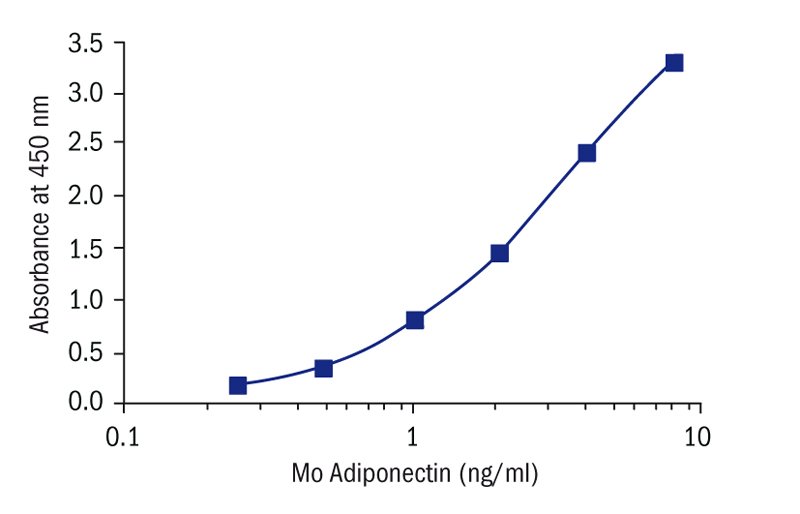
Calibration Curve
Calibration Range
0.25–8 ng/ml
Limit of Detection
0.079 ng/ml
Intra-assay (Within-Run)
n = 8; CV = 2.5%
Inter-assay (Run-to-Run)
n = 5; CV = 4.3%
Spiking Recovery
97,40%
Dilution Linearity
88,20%
Crossreactivity
- bovine Non-detectable
- cat Non-detectable
- dog Non-detectable
- goat Non-detectable
- hamster Non-detectable
- horse Non-detectable
- human Non-detectable
- monkey Non-detectable
- pig Non-detectable
- rabbit Non-detectable
- rat Non-detectable
- sheep Non-detectable
- chicken Not tested
- mouse Yes
Features
- The total assay time is less than 3 hours.
- The kit measures mouse serum Adiponectin/Acrp30.
- Quality Controls are mouse serum based.
- Components of the kit are provided ready-to-use, concentrated and lyophilized.
Research topic
Chronic renal failure, Coronary artery disease, Diabetology - Other Relevant Products, Energy metabolism and body weight regulation, Animal studies
Summary
Adiponectin, also referred to as Acrp30, AdipoQ and GBP-28, is a recently discovered 244
aminoacid protein, the product of the apM1 gene, which is physiologically active and
specifically and highly expressed in adipose cells (adipokine). The protein belongs to the
soluble defence collagen superfamily; it has a collagen-like domain structurally homologous
with collagen VIII and X and complement factor C1q-like globular domain. Adiponectin forms
homotrimers, which are the building blocks for higher order complexes found circulating in
serum. Adiponectin receptors AdipoR1 and AdipoR2 have been recently cloned; AdipoR1 is
abundantly expressed in skeletal muscle, whereas AdipoR2 is predominantly expressed in the
liver.
Paradoxically, adipose tissue-expressed adiponectin levels are inversely related to the degree
of adiposity. A reduction in adiponectin serum levels is accompanied by insulin resistance
states, such as obesity and type 2 diabetes mellitus. It is also reported in patients with coronary
artery disease. Increased adiponectin levels are associated with type 1 diabetes mellitus,
anorexia nervosa and chronic renal failure. Adiponectin concentrations correlate negatively
with glucose, insulin, triglyceride concentrations and body mass index and positively with highdensity
lipoprotein-cholesterol levels and insulin-stimulated glucose disposal.
Adiponectin has been shown to increase insulin sensitivity and decrease plasma glucose by
increasing tissue fat oxidation. It inhibits the inflammatory processes of atherosclerosis
suppressing the expression of adhesion and cytokine molecules in vascular endothelial cells
and macrophages, respectively. This adipokine plays a role as a scaffold of newly formed
collagen in myocardial remodelling after ischaemic injury and also stimulates angiogenesis by
promoting cross-talk between AMP-activated protein kinase and Akt signalling in endothelial
cells.
Find documents for the lot
Example Instructions for Use (RUO)
Example Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)
MSDS (RUO)