Cat # changed from RSCYK070R to YK070
Type
Competitive ELISA
Description
This EIA kit is used for qu antitative determination of CgA LI in human plasma and saliva samples .
The kit is characterized for sensitive quantification, high specificity and no influence with other
components in samples. CgA standard is highly purified synthetic product (purity over 98%) and the
content indicated is the absolute weight of the standard. N α biotinylglycylglycyl human
CgA(344 374)is used as labeled antigen which is proved stability.
Applications
Saliva, Plasma
Sample Requirements
25 µl
Shipping
On blue ice packs. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
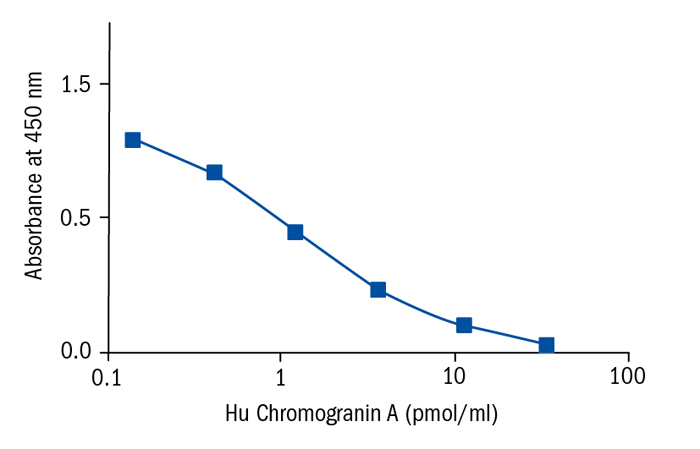
Calibration Curve
Calibration Range
0.14–33.33 pmol/ml
Intra-assay (Within-Run)
Plasma sample: 10.13 - 13.26 %
Saliva sample: 8.15-12.84 %
Inter-assay (Run-to-Run)
Plasma sample: 11.57-15.33 %
Saliva sample: 12.42 - 14.22 %
Spiking Recovery
Plasma sample: 126.02 %
Saliva sample: 96.6 %
Features
- RUO
- The assay kit can measure CgA in the range of 0.14 - 33.33 pmol/mL
- The assay is completed within 16 20 hr. + 2.5 hr
- With one assay kit, 41 samples can be measured in duplicate
- Intraassay CV (%) Plasma 10.13-13.26, saliva 8.15-12.84
- Interassay CV (%) Plasma 11.57-15.33, saliva 12.42-14.22
Research topic
Oncology
Summary
Chromogranin A (CgA) is an acidic secretory protein consisting of 439 amino acids in human. The
protein is found in a wide variety of hormone and neurotransmitter storage vesicles, and it is known
be co stored and co released with catecholamines from adrenal medulla and sympathet ic neuronal
vesicles during exocytosis. O Conner and Bernstein have first reported radioimmunological
measurement of CgA in human plasma under conditions of physiologic, pharmacologic and
pathologic alteration of sympathoadrenal function. Accumulated data, thereafter, have confirmed
high concentrations of plasma or serum CgA measured by radioimmunoassay (CgA like
immunoreactivity: CgA LI) in patients with neuroendocrine and endocrine tumors, especially in
those with pheochromocytoma, anterior pituitary tumo rs and rectal an d prostatic carcinoma. On the
other hand, Nakane et al. recently discovered that CgA LI exists in saliva, the concentration of
which elevates rapidly under psychosomatic stress even prior to the elevation of salivary cortisol
level. Subsequ ently, Kanno et al. presented an evidence for autonomic control of submandibular
CgA LI secretion in the anaesthetized rat.
Most of the reported measurement of CgA by radioimmunoassay utilized native CgA antigens
(full or partial length) and antibodies against the native proteins. On the other hand, Yanaihara et al.
provided a novel radioimmunoassay system for estimation of Cg A LI level in human plasma with
use of synthetic human CgA ( 344 374) and antibody raised against the synthetic peptide. Nakane et
al. also used the assay system in their work on human salivary CgA as mentioned above. CgA
molecules contain 9 10 sites of bas ic amino acid pairs (Arg Arg, Lys Arg, etc. etc.), which are generally
accepted as the proteolytic processing sites. In fact, the sequences corresponding to CgA derived
peptides having some biological activities, such as granin, pancreastatin and parastatin, in CgA
molecules are all preceded and followed by basic amino acid pairs. However, it is also known that in
the adrenal medulla which is the major site of CgA production, CgA is found to exist predominantly
in large molecular forms, supporting the least processing of CgA in adrenal chromaffin cells. In
addition, it was shown that there is no rapid degradation of the protein within the bl ood stream.
On the basis of these findings, Yanaihara Institute Inc. succeeded in developing, for the first time,
a specific, sensitive, stable and easy manipulative enzyme immunoassay (EIA) system for
measurement of human CgA LI using anti synthetic human CgA ( 344 374) antibody, synthetic
human CgA ( 344 374) as standard antigen and N α biotinylglycylglycyl human CgA ( 344 374) as
labeled antigen. The assay kit now available from the Institute can be used for measurement of
CgA LI in human biologi cal fluid such as plasma and saliva.
Find documents for the lot
Example Instructions for Use (RUO)
Example Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)