Cat # changed from RMEE20 to E20
Type
Sandwich ELISA, Biotin-labelled antibody
Description
The ELISA E20 is intended to be used for the measurement of human IGF-I in serum and plasma samples. In combination with growth retardation and other clinical symptoms the results of this test system can be used as supplementary data to assess disturbances of the growth hormone axis.
Applications
Serum, Plasma-EDTA, Plasma-Heparin, Plasma-Citrate
Sample Requirements
10 µl/well
Shipping
On blue ice packs. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the complete kit at 2–8°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
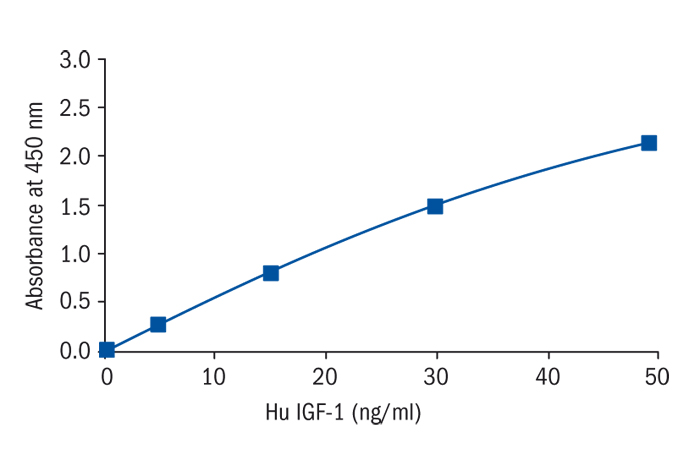
Calibration Curve
Calibration Range
2–50 ng/ml
Limit of Detection
0.091 ng/ml
Intra-assay (Within-Run)
CV = 5.81%
Inter-assay (Run-to-Run)
CV = 8.57%
Spiking Recovery
85-102%
Note
The kits are CE-IVD certified and intended for professional use.
Features
- *European Union: for in vitro diagnostic use
- Rest of the world: for research use only!
- duration: 1.75 h
- specific, monoclonal antibody and high-affinity polyclonal antiserum
- 2 Controls, freeze-dried
- 5 single Calibrators: 2 -50 ng/mL, recombinant human IGF-I
Research topic
Growth hormone and factor-related products
Summary
Insulin-like growth factors (IGF) I and II play a pivotal role in regulating the proliferation, differentiation and specific functions of many cell types (1-3). IGF-I is identical with Somatomedin C (Sm-C) (4) and has a molecular weight of 7649 Dalton (5). Its major regulators are growth hormone (GH) and nutrition (6), although its production in specific tissues is affected by a multitude of tropic hormones and other peptide growth factors. In contrast to many other peptide hormones, IGFs are avidly bound to specific binding proteins (IGFBP). The seven classes of IGFBPs which are known at present (7,8,22) either bind IGF-I and IGF-II with similar affinities or show a preference for IGF-II (9,10).
A major problem of IGF-I measurement results from the interference of IGFBPs in the assay. Direct determinations in untreated serum samples (11) give false values because of the extremely slow dissociation of the IGF-I/IGFBP-3 complexes during the assay incubation.
Therefore, various techniques were applied to physically separate IGF-I from its binding proteins before measurement, including (a) size exclusion chromatography under acidic conditions, (b) solid-phase extraction and (c) acid-ethanol extraction (2,12,13). These techniques, however, are either inconvenient or time-consuming or give incomplete and not-reproducible recoveries. The most widely used method is the acid-ethanol extraction (13,14) with a recovery of only 70-80 % of IGFBP-bound IGF-I as a result of co-precipitation. The absolute results of such an extraction are therefore false low (15). The extraction removes the IGFBPs only insufficiently and leads to reduction in sensitivity of the assay due to pre-dilution of the samples by the extraction procedure. Furthermore, the remaining IGFBP may still interfere in the assay. In addition, the acid-ethanol extraction is ineffective in specimens other than serum or plasma (e.g. cell culture media), in which determination of IGF-I is already difficult enough due to the fact that IGFBPs are frequently present at large excess.
To avoid these difficulties, an uncomplicated assay was developed, in which special sample preparation is not required before measurement.
Clinical Significance
There are apart from GH, a number of variables that influence serum IGF-I. Decreased levels are found in states of malnutrition/ malabsorption, hypothyroidism, liver disease, untreated diabetes mellitus, chronic inflammatory disease (1,6), malignant disease or polytrauma. High levels, on the other hand, are likely to be present in precocious puberty or obesity. Crucially important to the correct interpretation of IGF-I measurement is the relationship between age and IGF-I levels (see Table 2 and Fig.: 4-6).
Due to its GH-dependence, determination of serum IGF-I was shown to be a useful tool in diagnosis of growth disorders, especially with regard to GH deficiency (GHD) or acromegaly (6,16-19,23,24). The major advantage of IGF–I determination compared to GH determination is its stable circadian concentration; therefore a single measurement is sufficient. Hence IGF-I determination should be the first in a series of laboratory test. Clearly normal levels would then rule out disturbances of the GH-IGF-I-axis. Low levels, i.e. close to or below the age-related 5th percentile would indicate the necessity of further diagnostic efforts. Subnormal levels of IGF-I would be evidence for reduced GH secretion, if other causes of low serum IGF-I (e.g. malnutrition or impaired liver function) can be ruled out. For differentiation of healthy short children without GH deficiency and children with "classical" GH deficiency, the 0.1st percentile proved to be an appropriate cut-off point, especially after the age of eight. However, IGF-I levels of short children not suffering from GHD may nevertheless lay between the 0.1st and 5th percentile (19). In contrast, acromegaly is characterized by pathologically elevated IGF-I levels, which apparently reflect the severity of the disease better than GH-levels (17,18,20).
Find documents for the lot
Example Instructions for Use (RUO)
Example Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)
MSDS (RUO)
Product Brochure
CE IVD Assays
Other Documents
Declaration of Conformity