Type
Sandwich ELISA, Biotin-labelled antibody
Description
The human sVCAM-1 ELISA is an enzyme-linked immunosorbent assay for the quantitative detection of human sVCAM-1. The human sVCAM-1 ELISA is for research use only. Not for diagnostic or therapeutic procedures.
Applications
Serum, Plasma-EDTA, Plasma-Heparin, Plasma-Citrate, Amniotic fluid, Cell culture supernatant
Sample Requirements
100 µl (1:50 prediluted)
Shipping
On blue ice packs. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the kit at 2–8°C. The lyophilized controls at -20°C. Under these conditions, the kit is stable until the expiration date (see label on the box).
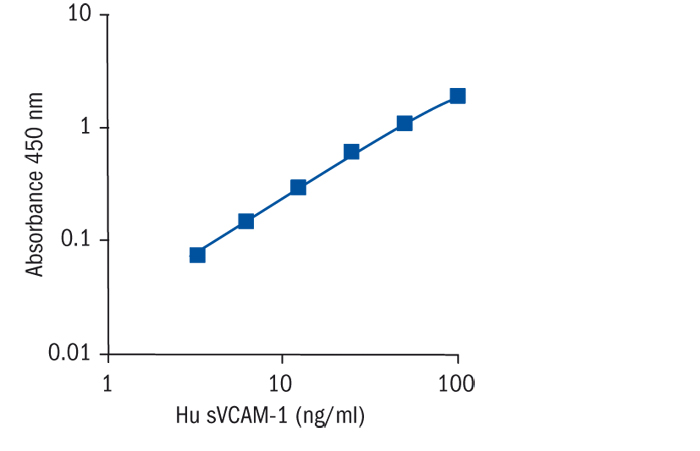
Calibration Curve
Calibration Range
3.1–100 ng/ml
Limit of Detection
0.6 ng/ml
Intra-assay (Within-Run)
CV = 3.1%
Inter-assay (Run-to-Run)
CV = 5.2%
Spiking Recovery
89,00%
Dilution Linearity
100,00%
Features
- RUO
- calibration range 3.1-100 ng/ml
- limit of detection 0.6 ng/ml
- lyophilized controls
- intra-assay CV = 3.1%
- inter-assay CV = 5.2%
Research topic
Cell adhesion proteins
Summary
The vascular cell adhesion molecule-1 (VCAM-1) or CD106 is a member of the immunoglobulin gene superfamily. The initial molecular cloning of VCAM-1 reported six extracellular Ig-like domains (6D VCAM-1).
This 6D VCAM-1 arises due to alternative splicing from a seven-domain VCAM-1 (7D VCAM-1). 7D VCAM-1 is the dominant form expressed by cultured human endothelial cells. Domains 1 through 3 are highly homologous to domains 4 through 6, suggesting that they arose by gene duplication. The cDNA of 7D VCAM-1 predicts a core protein of approximately 81 kDa with seven potential N-linked glycosylation sites. Upon complete glycosylation the mature protein has a molecular weight of approximately 102 kDa. This observation is in general agreement with immunoprecipitation studies that show a protein of approximately 110 kDa on cytokine-activated endothelium.
Murine and rat VCAM-1 have been cloned. In contrast to
ICAM-1, VCAM-1 appears to have been highly conserved through evolution. Both rat and mouse VCAM-1 are highly homologous at the protein level to the human VCAM-1 (77% and 76%, respectively).
VCAM-1 supports the adhesion of lymphocytes, monocytes, natural killer cells, eosinophils, and basophils through its interaction with leukocyte very late antigen-4 (VLA-4). VCAM-1/VLA-4 interaction mediates firm adherence of circulating non-neutrophilic leukocytes to endothelium. VCAM-1 also participates in leukocyte adhesion outside of the vasculature, mediating precursor lymphocyte adhesion to bone marrow stromal cells and B cell binding to lymph node follicular dendritic cells.
VCAM-1 is not constitutively expressed on endothelium, but can be up-regulated in vitro in response to LPS, TNF-, and IL-1, as well as to interferon- and IL-4.
VCAM-1 is also present on tissue macrophages, dendritic cells, bone marrow fibroblasts, myoblasts and myotubes.
Find documents for the lot
Example Instructions for Use (RUO)
Example Instructions for Use (RUO)
Safety Information (RUO)
MSDS (RUO)