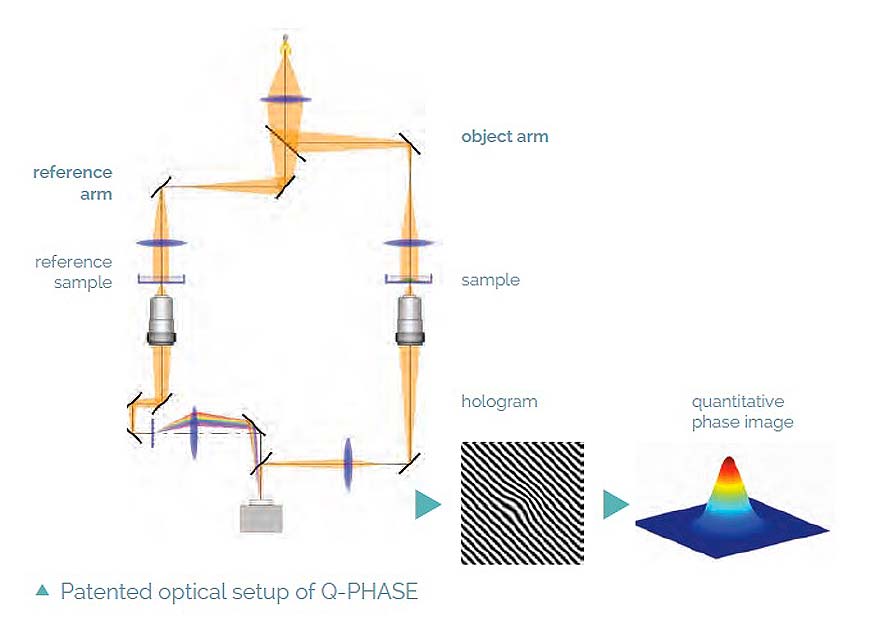
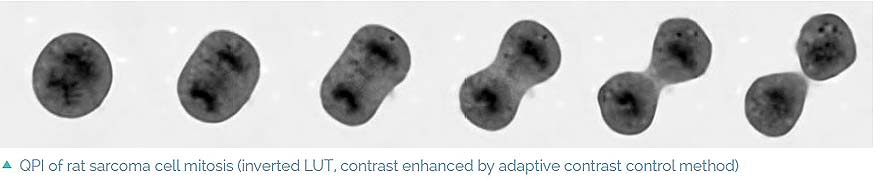
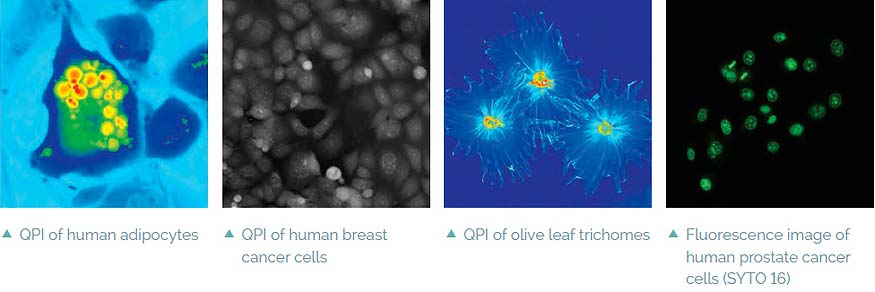
- No image artifacts such as halo effect (as opposed to techniques based on Zernike phase contrast illumination)
- Enables very precise detection of cell boundaries
- Strong suppression of coherent noise (speckles) & parasitic interferences
- (as opposed to laser-based approaches)
- Label-free – no staining is needed, simple sample preparation, observation
- of live cells in their native environment, no photobleaching problems
- Low phototoxicity – low light power density (107× lower than fluorescence
- microscopy) allows long-term observations (for days)
- Coherence-gating effect – Q-PHASE special feature enables it to observe samples
- even in scattering media (phospholipid emulsions, extracellular matrices, etc.)
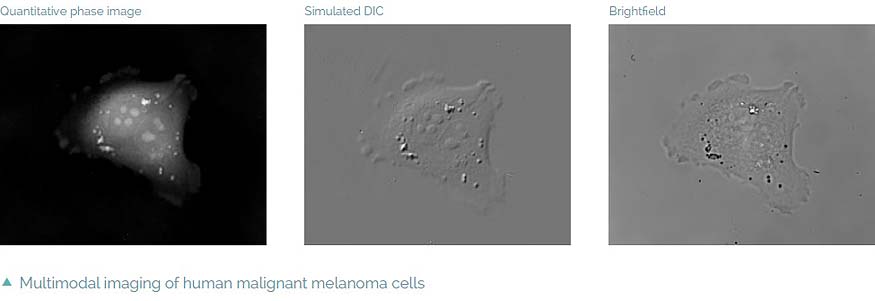
- Multimodality – fully integrated fluorescence module, simulated DIC and
- brightfield which enables automatic multimodal imaging of the sample
- High-quality QPI – unique Q-PHASE’s optical setup allows using incoherent illumination
- which provides extraordinary imaging quality without any compromises
- Lateral resolution of conventional microscopes (up to 2× better when compared to common laser-based approaches or pinhole spatial filtering based techniques)
- Fast acquisition – the use of off-axis holographic approach makes Q-PHASE a single-shot instrument, thus enabling imaging of very fast cell dynamics
- Full motorization – focusing, sample stage, objective exchange, fluorescence
- filters
- Automated multidimensional acquisition – time-lapse, channel, position, Z-stack
- Simple image segmentation and processing – comparable to fluorescence data processing
- Quantitative – phase values can be recalculated (e.g. to cell dry-mass density (pg/μm2) or direct topography) with nanometer sensitivity (usually non-biological samples with homogeneous refractive index distribution)
- High phase detection sensitivity – is able to detect even the smallest changes in axial direction, very sensitive detection of morphology or position changes
Fluorescence module
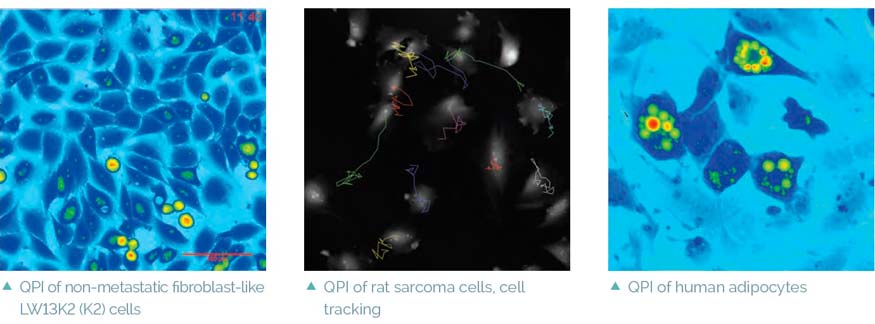
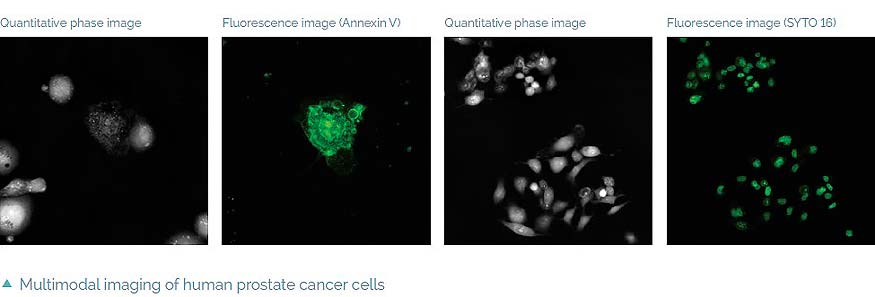
The Q-PHASE can combine holographic microscopy with fluorescence microscopy. This powerful combination provides the possibility to verify structures or processes observed in QPI with fluorescence microscopy in the same field of view using a single instrument. For example, morphological and position changes prior to cell death can be observed in QPI followed by fluorescence verification of cell death types (see images below) [4]. This approach greatly reduces the phototoxicity and photobleaching problems of fluorescence imaging and it allows long-term observations.
The focus plane in both methods is located at the same position. This allows easy and fast switching between the two imaging methods at the same conditions and time points. Multiple fluorescence channels are possible with motorized channel exchange for automated multidimensional measurements.
The illumination can be implemented by using liquid light guide coupled solid state light sources or a xenon arc lamp. Multidimensional image acquisition combining holography and fluorescence is fully integrated in the Q-PHASE’s software. A fluorescence module is attached to the side port of the Q-PHASE, which can alternatively be used for other imaging techniques.
Intrinsic imaging modes
Complementary image contrast can be obtained simply by numerical processing of the acquired phase images. In this way simulated DIC images can be produced with adjustable shear and displayed in real time. Another possibility is brightfield imaging which can be simply achieved by closing the reference arm of the microscope. In summary, the Q-PHASE offers multiple imaging modes widely used in biological research such as fluorescence or DIC integrated in a single instrument and supported by the Q-PHASE’s software allowing fully automated multimodal imaging.
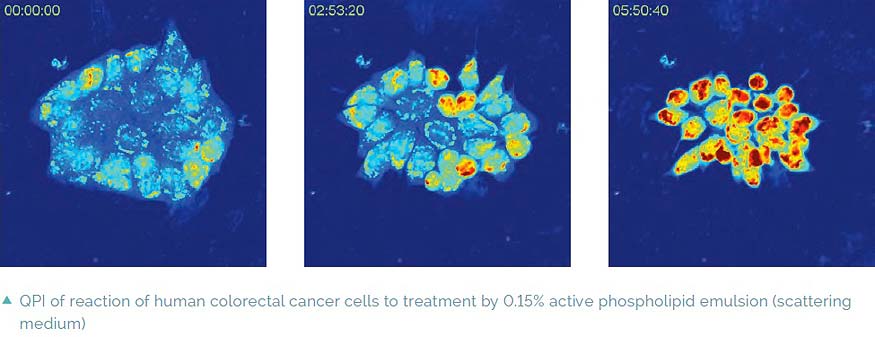
Imaging in scattering media
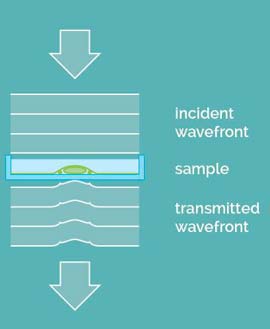
A special feature of Q-PHASE is coherence-gating, a well-known effect in optical coherence tomography which enables observations of samples even in scattering media. This effect is induced by using incoherent light in the unique patented setup of Q-PHASE. Its transmitted-light configuration enables it to effectively suppress the light which was scattered by the environment in defocused planes and to only use unscattered light for imaging. In this way, cells can be observed even in moderately scattering non-transparent substances such as an active phospholipid emulsion.
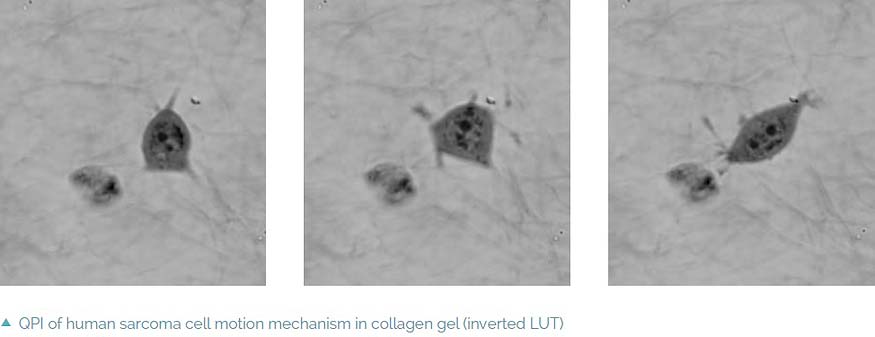
Imaging in extracellular matrices
The coherence-gating effect can also be beneficial when imaging cells in extracellular matrices such as collagen gel. Extracellular matrices mimic an in vivo environment making the study of the cell’s dynamic reactions to its surroundings more realistic. Usually it is used as a biological test for cancer cell invasivity and ability to metastasize. The Q-PHASE microscope enables one to record a mechanism of cell motion and interactions between extracellular matrix fibers and cells with high contrast and without any additional staining.