Type
Native protein
Description
Native protein purified from human plasma
Source
Plasma
Purity
˃ 90 % by SDS-PAGE
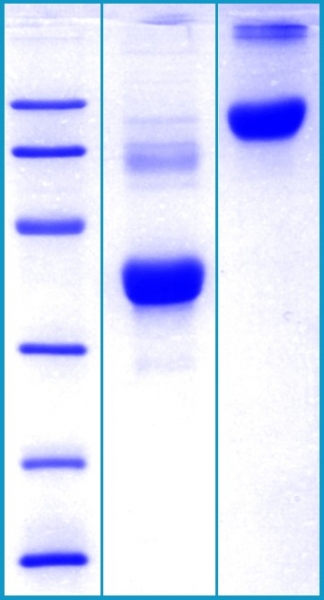
SDS-PAGE Gel
12% SDS-PAGE separation of Human Clusterin
1. M.W. marker – 14, 21, 31, 45, 66, 97 kDa
2. reduced and heated sample, 5μg/lane
3. non-reduced and non-heated sample, 5μg/lane
Endotoxin
< 1.0 EU/ug
Formulation
Filtered (0.4 μm) and lyophilized in 0.5 mg/mL in PBS buffer, pH 7.5
Reconstitution
Add deionized water to prepare a working stock solution of 0.5 mg/mL and let the lyophilized pellet dissolve completely.
Applications
Western blotting, ELISA
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the lyophilized protein at -80 °C. Lyophilized protein remains stable until the expiry date when stored at -80 °C. Aliquot reconstituted protein to avoid repeated freezing/thawing cycles and store at -80 °C for long term storage. Reconstituted protein can be stored at 4 °C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
Endotoxin level determination.
Note
Material used for protein preparation was tested and found negative for anti-HCV and HIV Ag/Ab. Since no test can absolutely assure the absence of all infectious agents, this product should be handled as a potential biohazard. This product is intended for research use only.