Type
Recombinant protein
Description
Total 174 AA. MW: 19.12 kDa (calculated). UniProtKB acc.no. P62937 (Val2-Glu165). N-Terminal His-tag (10 extra AA). Protein identity confirmed by MS.
Amino Acid Sequence
MKHHHHHHASVNPTVFFDIAVDGEPLGRVSFELFADKVPKTAENFRALSTGEKGFGYKGSCFHRIIPGFMCQGGDFTRHNGTGGKSIYGEKFEDENFILKHTGPGILSMANAGPNTNGSQFFICTAKTEWLDGKHVVFGKVKEGMNIVEAMERFGSRNGKTSKKITIADCGQLE
Source
E. coli
Purity
˃ 90 % by SDS-PAGE
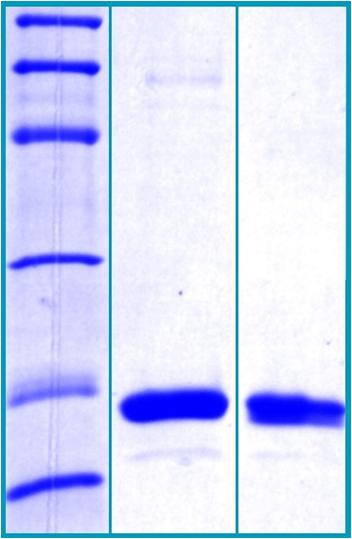
SDS-PAGE Gel
14% SDS-PAGE separation of Hu Cyclophilin A:
1. M.W. marker – 97, 66, 45, 31, 21, 14 kDa
2. reduced and heated sample, 2.5μg/lane
3. non-reduced and non-heated sample, 2.5μg/lane
Endotoxin
< 1.0 EU/µg
Formulation
Filtered (0.4 μm) and lyophilized from 0.5 mg/mL in 20mM Tris buffer, 50 mM NaCl, pH 7.0
Reconstitution
Add 200µl of deionized water to prepare a working stock solution of approximately 0.5 mg/ml and let the lyophilized pellet dissolve completely.
Applications
Western blotting, ELISA
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the lyophilized protein at -80 °C. Lyophilized protein remains stable until the expiry date when stored at -80 °C. Aliquot reconstituted protein to avoid repeated freezing/thawing cycles and store at -80 °C for long term storage. Reconstituted protein can be stored at 4 °C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
Endotoxin level determination.
Note
This product is intended for research use only.
Research topic
Cardiovascular disease, Immune Response, Infection and Inflammation, Oncology
Summary
Cyclophilins belong to a group of proteins that have peptidyl-prolyl cis-trans isomerase activity; such proteins are collectively known as immunophilins and also include the FK-506-binding proteins and the parvulins. Cyclophilins are found in all cells of all organisms studied, in both prokaryotes and eukaryotes. Human have a total of 16 cyclophilin proteins. Cyclophilins also have varying degrees of affinity for the immunosuppressive drug Cyclosporine A (CsA), a cyclic 11-amino-acid peptide produced by fungus Tolypocladium infantum. Cyclophilin A, in particular, is the major intracellular receptor for CsA. Cyclophilin A (CYPA) is the first member of the cyclophilins to be identified in mammals. Human genes of CYPA, also known as Cyp18, are located on chromosome 7p11.2-p13 and encode the protein, which consists of 165 amino acid residues with a relative molecular mass approximately 18 kDa. Human CYPA has an eight-stranded antiparallel β-barrel structure, with two α-helices enclosing the barrel from either side. Seven aromatic and other hydrophobic residues form a compact hydrophobic core within the barrel, usually in the area where CsA binds. A loop from Lys118 ti His126 and four β-strands (β3-β6) make up the binding site for CsA. In mammals, the CsA-CYPA complex binds to and inhibits calcineurin, a calcium-calmodulin-activated serine/threonine-specific protein phosphatase. The inhibition of calcineurin blocks the translocation of nuclear factor of activated T cells from the cytosol to the nucleus, thus preventing the transcription of genes encoding cytokines such as interleukin-2. Extracellular CYPA has a potent chemotactic effect on leukocytes, monocytes, and lymphocytes. CYPA is believed to be a key molecule in many biological functions including molecular chaperoning, protein folding, protein trafficking, immune modulation and cell signaling. Secreted CYPA activates cardiovascular cells resulting in a variety of cardiovascular diseases, including vascular remodeling, abdominal aortic aneurysms formation, atherosclerosis, cardiac hypertrophy and myocardial ischemic reperfusion injury. Intracellular CYPA is secreted from cells in response to inflammatory stimuli such as hypoxia, infection and oxidative stress. Its expression increases in inflammatory conditions including rheumatoid arthritis, autoimmune disease and cancer. CYPA levels are higher in the serum and synovial fluids of rheumatoid arthritis patients and are also elevated in tumors including non-small cell lung cancer, pancreatic adenocarcinoma, hepatocellular carcinoma, oral cancer, buccal squamous cell carcinomas and breast cancer.