Type
Recombinant protein
Description
Total 346 AA. MW: 38.3 kDa (calculated). UniProtKB acc.no. P29279. N-Terminal His-tag and Xa – cleavage site, 23 extra AA.
Amino Acid Sequence
MGHHHHHHHHHHSSGHIEGRHMRQNCSGPCRCPDEPAPRCPAGVSLVLDGCGCCRVCAKQLGELCTERDPCDPHKGLFCDFGSPANRKIGVCTAKDGAPCIFGGTVYRSGESFQSSCKYQCTCLDGAVGCMPLCSMDVRLPSPDCPFPRRVKLPGKCCEEWVCDEPKDQTVVGPALAAYRLEDTFGPDPTMIRANCLVQTTEWSACSKTCGMGISTRVTNDNASCRLEKQSRLCMVRPCEADLEENIKKGKKCIRTPKISKPIKFELSGCTSMKTYRAKFCGVCTDGRCCTPHRTTTLPVEFKCPDGEVMKKNMMFIKTCACHYNCPGDNDIFESLYYRKMYGDMA
Source
E. coli
Purity
˃ 90 % by SDS-PAGE
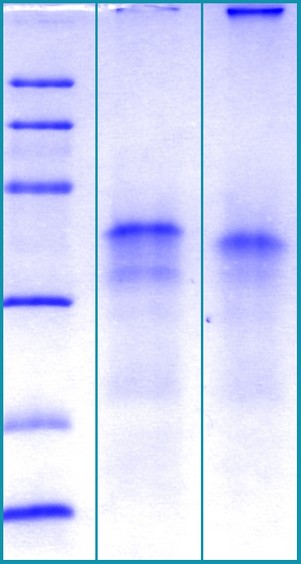
SDS-PAGE Gel
12% SDS-PAGE separation of Human CTGF
1. M.W. marker – 14, 21, 31, 45, 66, 97 kDa
2. reduced and heated sample, 2.5μg/lane
3. non-reduced and non-heated sample, 2.5μg/lane
Endotoxin
< 1.0 EU/µg
Formulation
Filtered (0.4 μm) and lyophilized in 0.5 mg/mL in 0.05 M Acetate buffer pH=4.0; 4% mannitol, 1% sucrose
Reconstitution
Add 0.1M Acetate buffer pH=4.0 to prepare a working stock solution of 0.5 mg/mL and let the lyophilized pellet dissolve completely at 37°C. For conversion into higher pH value, we recommend intensive dilution by relevant buffer to a concentration of 10μg/mL. In higher concentrations the solubility of this antigen is limited.
Applications
Western blotting, Cell culture and/or animal studies
Shipping
On ice. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
Store the lyophilized protein at -80 °C. Lyophilized protein remains stable until the expiry date when stored at -80 °C. Aliquot reconstituted protein to avoid repeated freezing/thawing cycles and store at -80 °C for long term storage. Reconstituted protein can be stored at 4 °C for a week.
Quality Control Test
BCA to determine quantity of the protein.
SDS PAGE to determine purity of the protein.
Endotoxin level determination.
Note
This product is intended for research use only.
Research topic
Apoptosis, Bone and cartilage metabolism, Cardiovascular disease, Coronary artery disease, Extracellular matrix, Oncology, Others, Pulmonary diseases, Renal disease
Summary
Connective Tissue Growth Factor belongs to the CCN family of proteins. The CCN family presently consists of six members in human also known as: Cyr61 (Cystein rich 61), CTGF (Connective Tissue Growth Factor), Nov (Nephroblastoma Overexpressed gene), WISP-1, 2 and 3 (Wnt-1 Induced Secreted Proteins). The CCN genes encode secreted proteins associated with the Extracellular Matrix (ECM) and cell membrane. CCN proteins are matricellular proteins which are involved in the regulation of various cellular functions including: proliferation, differentiation, survival, adhesion and migration. They are expressed in derivatives of the three embryonic sheets and are implicated in the development of kidney, nervous system, muscle, bone marrow, cartilage and bone. During adulthood, they are implicated in wound healing, bone fracture repair, and pathologies such as: fibrosis, vascular ailments and tumourigenesis. Full length secreted CCN proteins can show an antiproliferative activity, whereas truncated isoforms are likely to stimulate proliferation and behave as oncogenes. The full-length protein consists of four modules: - Module I shares partial identity with the N-terminal part of the Insulin-like Growth Factor Binding Proteins (IGFBPs). - Module II includes a stretch of 70amino acid residues – which shares sequence identity with the Von Willebrand Factor Type C repeat (VWC). - Module III contains sequences sharing identity with the Thrombospondin type 1 repeat (TSP1) (WSXCSXXCG), which is thought to be implicated in the binding of sulfated glycoconjugates and to be important for cell adhesion. - Module IV, also designated CT, is encoded by exon 5. It is the least conserved one of the four domains at the level of nucleotide sequence, but it appears to be critical for several of the biological functions attributed to the CCN proteins. Module IV resembles the CT domain of several extracellular protein including, Von Willebrand's factor and mucins. Sequence similarities to heparin-binding motifs are also found within this domain. Proteolysis of the secreted full-length CCN proteins that has been reported in the case of CCN2 and CCN3 might result in the production of CCN-derived peptides with high affinity for ligands that full-length CNN proteins bind only poorly. Amino-truncated CCN2 isoforms were biologically active whereas no specific biological activity has been attributed to the truncated CCN3. Although the molecular processes underlying the production of these secreted isoforms is presently unknown, it is important to note that proteolysis occur at the same amino acid residues in both CCN2 and CCN3. An elevated expression of CCN2 has also been detected by Northern blotting in human invasive mammary ductal carcinomas, dermatofibromas, pyogenic granuloma, endothelial cells of angiolipomas and angioleiomyomas, and in pancreatic tumours. A study performed with chondrosarcomas representative of various histological grades established that CCN2 expression was closely correlated with increasing levels of malignancy.In agreement with CCN2 playing a role in brain tumour angiogenesis, immunocytochemistry studies indicated that both glioblastoma tumour cells and proliferating endothelial cells stained positive for CCN2. In astrocytomas, CCN2 expression was particularly elevated in high-grade tumours, with a marked effect of CCN2 on cell proliferation. Downregulation of CCN2 expression in these cells was associated with a growth arrest at the G1/S transition while over-expression of CCN2 induced a two-fold increase of the number of cells in the G1 phase. Gene profiling analysis allowed to identify a set of about 50 genes whose expression might account for the proliferative activity of CCN2 in these cells. CCN2 was seen in a higher proportion of mononuclear cells of patients with acute lymphoblastic leukemia.