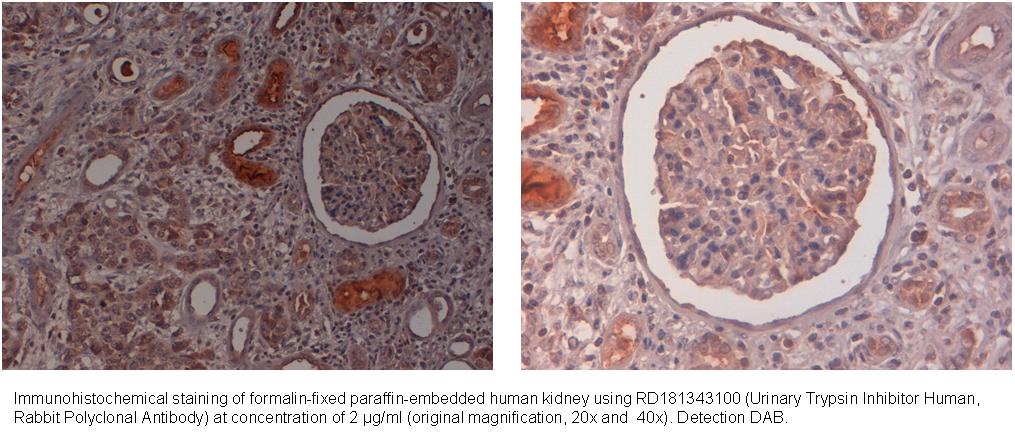
Type
Polyclonal Antibody
Applications
Western blotting, ELISA, Immunohistochemistry
Antibodies Applications
Source of Antigen
Human urine
Hosts
Rabbit
Preparation
The antibody was raised in rabbits by immunization with Human Urinary Trypsin Inhibitor.
Species Reactivity
Human. Not yet tested in other species.
Purification Method
Immunoaffinity chromatography on a column with immobilized Human Urinary Trypsin Inhibitor.
Antibody Content
0.1 mg (determined by BCA method, BSA was used as a standard)
Formulation
The antibody is lyophilized in 0.05 M phosphate buffer, 0.1 M NaCl, pH 7.2.
Reconstitution
Add 0.2 ml of deionized water and let the lyophilized pellet dissolve completely. Slight turbidity may occur after reconstitution, which does not affect activity of the antibody. In this case clarify the solution by centrifugation.
Shipping
At ambient temperature. Upon receipt, store the product at the temperature recommended below.
Storage/Expiration
The lyophilized antibody remains stable and fully active until the expiry date when stored at -20°C. Aliquot the product after reconstitution to avoid repeated freezing/thawing cycles and store frozen at -80°C. Reconstituted antibody can be stored at 4°C for a limited period of time; it does not show decline in activity after one week at 4°C.
Quality Control Test
Indirect ELISA – to determine titer of the antibody
SDS PAGE – to determine purity of the antibody
BCA - to determine quantity of the antibody
Note
This product is for research use only.
Research topic
Immune Response, Infection and Inflammation, Oncology, Renal disease, Sepsis
Summary
Urinary trypsin inhibitor (UTI) (also called bikunin or ulinastatin) is a multivalent serine protease inhibitor synthesized and released in human urine and blood. UTI is an acidic glycoprotein, composed of 143-amino acid residues. Bikunin contains two proteinase inhibitor domains of the Kunitz type, a short connecting peptide as well as N- and C-terminal extensions 10 – 25 amino acids long. The N-terminal extension carries a chondroitin sulphate chain. Each of the Kunitz domains has a binding site for a proteinase and the amino acid residues essential for binding (Met 36 of the N-terminal domain and Arg 92 of the C-terminal domain). The total molecular weight of UTI is 25 – 26 kDa. UTI is produced by endoplasmic reticulum of hepatocytes as a precursor in which UTI is linked to α1-microglobulin. Most of the UTI in blood (90 – 98 %) occurs as a covalently linked subunit of the proteins pre-α-inhibitor and inter-α-inhibitor, respectively. In human plasma the major UTI-containing protein is inter-α-inhibitor. The total concentration of UTI in human plasma is 4 – 7 μM, of which 2 – 10 % is in free form. UTI is a positive acute phase protein. The concentration of free, uncomplexed UTI in plasma of patients with inflammatory conditions has been reported to be higher than normal. The plasma UTI level and its gene expression change under severe inflammatory conditions. In patients suffering from various nephropathies, a clear correlation between the UTI and creatinine concentrations in plasma was found, implying that the kidneys are a major site of uptake of the protein. UTI is rapidly released into urine when infection occurs and is an excellent inflammatory marker, constituting most of the urinary anti-trypsin activity. In urine, in which the level of complexed UTI is negligible, the average UTI concentration is 0.03 – 0.05 μM. The level of UTI in urine may be elevated under various pathological conditions, including pneumonia, lung emphysema, rheumatoid arthritis, cancer, and surgical trauma. It appears that UTI passes through the kidneys by glomerular filtration. Some tumour cells secrete UTI, which could contribute to the high urinary levels seen in some cancer patients. The function of UTI has been preserved during evolution. Trypsin and other serine proteases such as trombin, chymotrypsin, kallikrein, plasmin, neutrophil elastase, cathepsin and factors IXa, Xa, XIa and XlIa are inhibited by UTI, indicating that UTI is part of the inflammatory process. Furthermore, UTI can suppress urokinase-type plasminogen activator (uPA) expression through the inhibition of protein kinase C. UTI appears to prevent organ injury by inhibiting the activity of inflammatory serine proteases. In vitro studies have demonstrated that serine protease inhibitors may have anti-inflammatory properties. UTI suppresses the infiltration of neutrophils and the release of elastase and chemical mediators from them. Clinically, UTI is widely used as a drug for patients with acute inflammatory disorders such as pancreatitis, shock and disseminated intravascular coagulation.