Update 2026: miREIA assay kits have been discontinued as of January 1, 2026.
The microRNA project now continues exclusively on the Two-Tailed RT-qPCR (TT-PCR) platform.
Acute coronary syndromes (ACS) are major causes of morbidity and mortality. Despite optimal medical treatment and revascularisation there remains a significant risk of subsequent cardiovascular events.
The central process underlying ACS is the development of a thrombus overlying a ruptured or eroded plaque, leading to various degrees of acute vessel occlusion and myocardial ischaemia. A thrombus that originates following plaque rupture consists largely of platelets; in addition, coagulation pathways are also triggered by plaque rupture and platelet aggregation.
Early mechanical and chemical restoration of blood flow through ischemic tissue and the use of anticoagulant agents respectively form the basis of ACS treatment.
It has been proven that these measures reduce the frequency of both early and late cardiovascular events including myocardial infarction and stent thrombosis, as well as a reduction in mortality.
In its most recent definition of myocardial infarction (MI), the European Society of Cardiology has refined approaches to classify and differentiate MI. While higher sensitivity troponin assays have improved the identification of low-risk patients suitable for immediate discharge, detecting and treating minor cardiac damage may fail to result in better clinical outcomes. There is still a need for biomarkers that facilitate early rule-out/rule-in of clinically relevant myocardial infarction.
Recently, noncoding RNAs (ncRNAs) have been implicated as biomarkers of myocardial infarction. MicroRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs) are among the ncRNAs present in the circulation. Plasma and serum levels of muscle- and cardiac-enriched miRNAs increase markedly after myocardial infarction.
Therefore miRNAs, lncRNAs, and circRNAs have attracted interest as potential biomarkers in cardiovascular disease.
Levels of the long intergenic ncRNA predicting cardiac remodeling were reported to predict adverse cardiac remodeling and death after myocardial infarction. However, circRNAs are less susceptible to RNase activity and may offer tissue specificity.
In addition, more than 15,000 cirRNAs are present in the human heart.
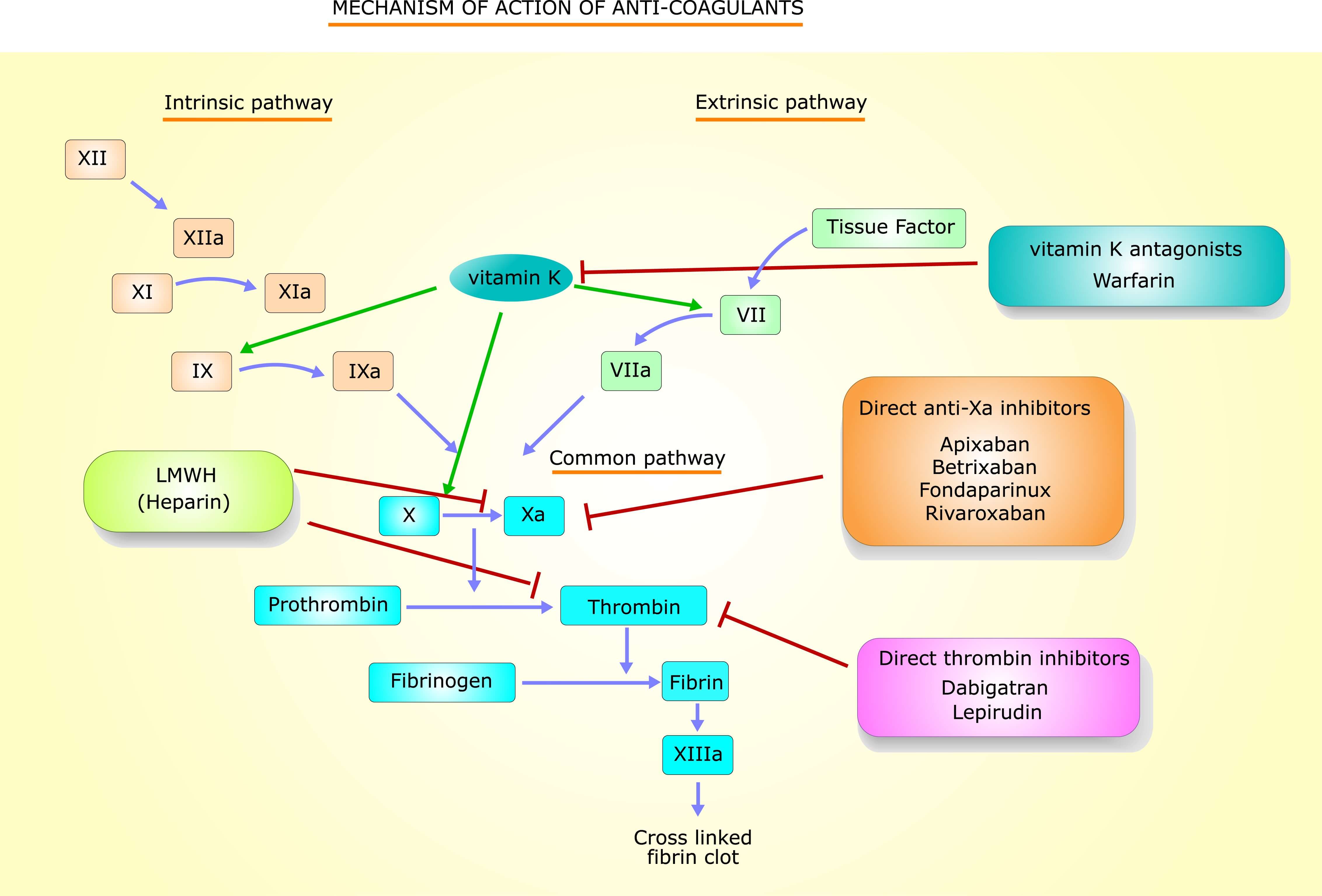
Heparin, also known as unfractionated heparin is a naturally occuring glycosaminoglycan and a medication. As a medication it is used as an anticoagulant commonly administered in the clinical setting of myocardial infarction.
Unfortunately, Heparin is a major confounding factor for measurements of ncRNAs by real-time polymerase chain reaction (qPCR). Few studies on the release of ncRNAs after MI used heparinase treatment to overcome the confounding effect by heparin.
Moreover, while circulating levels of muscle- and cardiac-enriched miRNAs have been shown to correlate to troponins after myocardial infarction, differences in the release of ncRNAs and novel protein biomarkers such as cardiac myosin-binding protein C (cMyBP-C) have not been compared in the most clinically relevant setting of MI patients presenting early with low troponin values.
Neither release kinetics of noncoding RNAs without confounding by heparin, nor the relationship of ncRNA to myocardial protein biomarkers have been exlored.
Researcher Manuel Mayr and colleagues of the King’s British Heart Foundation Centre, in collaboration with scientists from Bart’s Heart Centre, University Heart Centre Hamburg Eppendorf, and German Centre of Cardiovascular Research, were interested in comparing different classes of ncRNAs with cardiac protein.
The objective of their study was to use heparinase treatment to establish the release kinetics of three different types of noncoding RNAs (miRNAs, lncRNAs, and circRNAs) in serial samples.
They examined more than 2500 samples from patients undergoing transcoronary ablation of septal hypertrophy (TASH) as well as in patients with acute myocardial infarction presenting with a wide range of hs-cTn (high-sensitive cardiac troponin) levels.
The performance of ncRNA was compared with hs-cTn and cMyBP-C as established and emerging cardiac injury protein biomarkers.
Their screening of 21 lncRNAs and 158 circRNAs in human cardiac tissue identified 12 circRNAs and 11 lncRNAs as potential biomarkers with cardiac origin. Eleven miRNAs were also included.
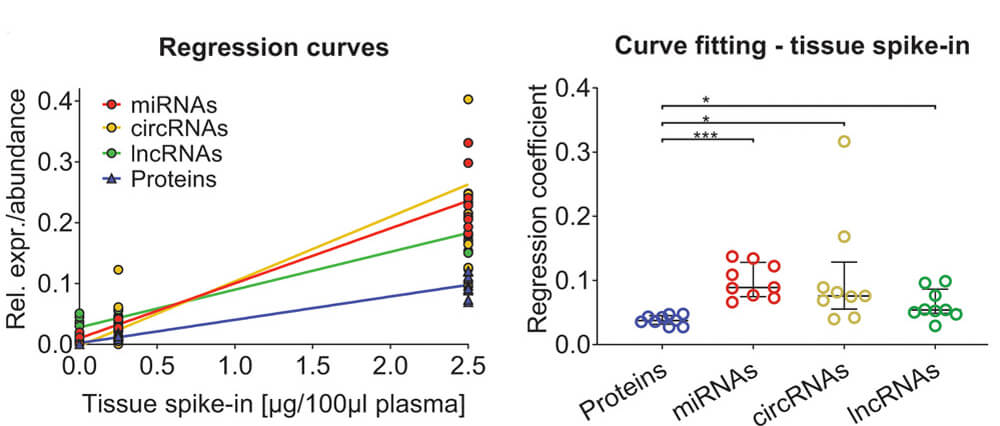
It was interesting that at low spike-in concentrations of myocardial tissue, significantly higher regression coefficients were observed across noncoding RNAs types compared with cardiac troponins and cMyBP-C.
Proteomics experiments have shown that cMyBP-C is released earlier upon myocardial ischemia than cardiac troponins and may contribute to a better rule-out/rule-in classification of myocardial infarction.
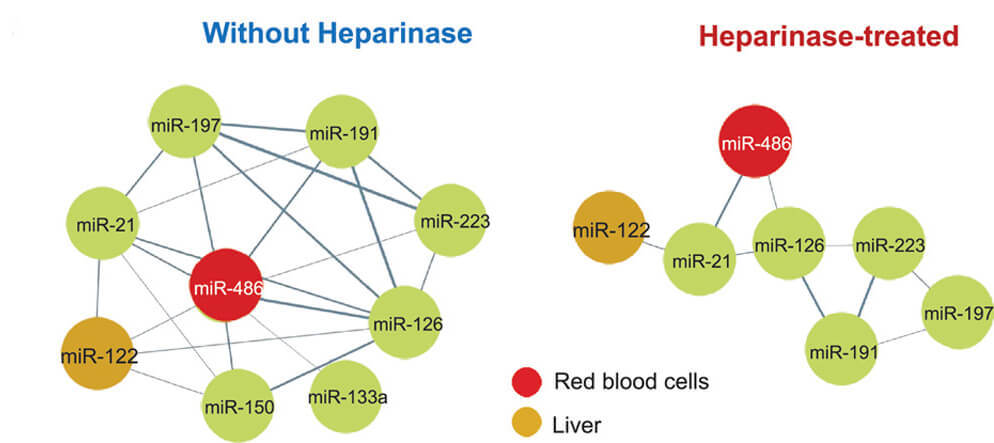
It was noteworthy that heparinase treatment of serial plasma and serum samples of patients undergoing transcoronary ablation of septal hypertrophy removed spurious correlations between miRNAs in non-heparinase-treated samples.
The following illustrations show clustering networks of miRNAs in the transcoronary ablation of septal hypertrophy cohort. Before heparinase treatment the analyzed miRNAs showed a dense correlation network. Heparinase treatment resolved this clustering of miRNAs, removing spurious correlations between miRNAs in non-heparinase-treated samples. The distinct cellular origin of miRNAs, ie, liver-specific miR-122 vs red blood cell-associated miR-486, became more readily apparent.
After transcoronary ablation of septal hypertrophy, muscle enriched miRNAs (miR-1 and miR-133a) showed a steeper and earlier increase than cardiac-enriched miRNAs (miR- 499 and miR-208b).
Cardiac myosin-binding protein C was validated as a biomarker with highly sensitive properties, and this combination with muscle-enriched miRNAs and with high-sensitive cardiac troponin T yielded the highest area under the curve values.
The results of the study clearly show that the sensitivity of current microRNA detection is inferior to cardiac proteins but miRNAs emerged as promising candidates to integrate noncoding RNAs with protein biomarkers.
